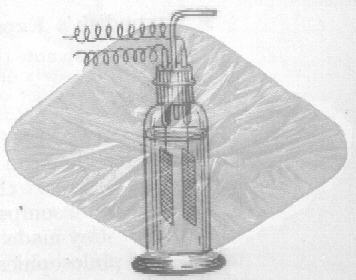


First prepared by G.Gore in 1858 by simple electrolysis, this substance is a solid solution of antimony trichloride in an unstable, allotropic form of antimony. It is prepared by the slow electrolysis of a concentrated solution of antimony trichloride in hydrochloric acid. The anode is of antimony metal, the cathode of platinum or copper. The explosive antimony (alpha-antimony allotrope with between 4 and 20% antimony trichloride) is deposited on the cathode and, in appearance, looks like polished graphite. If the deposit is scratched, the meta-stable allotrope is violently converted into the stable allotrope (common, or beta antimony) with the evolution of heat. The antimony trichloride held as a solid solution is vapourised (b.p. 223.5), and the explosion is therefore accompanied by the evolution of white clouds of the trichloride.
Similar phenomenon can be observed by the electrolysis of the tribromide and tri-iodide. Here are a couple of extracts about antimony in which the explosive allotrope is mentioned (large graphics files).